
The laboratory has long been the beating heart of life science innovation. White coats, pipettes, Petri dishes, and test tubes have defined the work of discovery for centuries. Today, however, a quiet revolution is reshaping the very idea of what it means to “do science.” Artificial intelligence (AI) is not just supporting lab research—it is increasingly becoming the lab itself. Algorithms are simulating biological processes, predicting experimental outcomes, and in some cases replacing traditional bench work altogether.
This convergence of AI and lab science is creating profound implications for how we discover drugs, develop diagnostics, and design therapies. While we have a long way before AI takes over, there are significant changes in process thanks to AI, especially in early, pre-clinical research impacts that are faster and also require fewer team members. For early-stage investors in life sciences, the opportunities are enormous—but so are the risks of betting on the wrong side of this technological transformation.
The Rise of the In-Silico Lab
Until recently, the gold standard of experimentation was the physical assay. If a company wanted to know whether a new compound might bind to a cancer receptor, it had to be synthesized, purified, and tested in a cell culture or animal model. This process often took months, consumed millions of dollars, and still yielded inconclusive or irreproducible results.
Enter the in-silico lab. By combining massive biological datasets with machine learning models, researchers can now simulate chemical interactions and biological pathways with increasing accuracy. Instead of making and testing thousands of molecules, scientists can computationally model which dozen are most likely to succeed before moving to physical validation.
For example:
- DeepMind’s AlphaFold stunned the scientific world by predicting the 3D structures of over 200 million proteins, something that once required painstaking crystallography.
- Insilico Medicine and Recursion Pharmaceuticals are using AI to design drug candidates and predict biological responses, often skipping years of benchwork.
- Startups are even developing “digital twins” of cells and organs, allowing in-silico experiments on virtual biology.
We are not yet at the point where AI makes Petri dishes obsolete, but we are undeniably seeing cases where algorithms narrow the experimental funnel so dramatically that physical biology becomes a confirmatory step rather than the exploratory foundation.
Three Major Implications of AI in the Lab
1. Radical Acceleration of Discovery
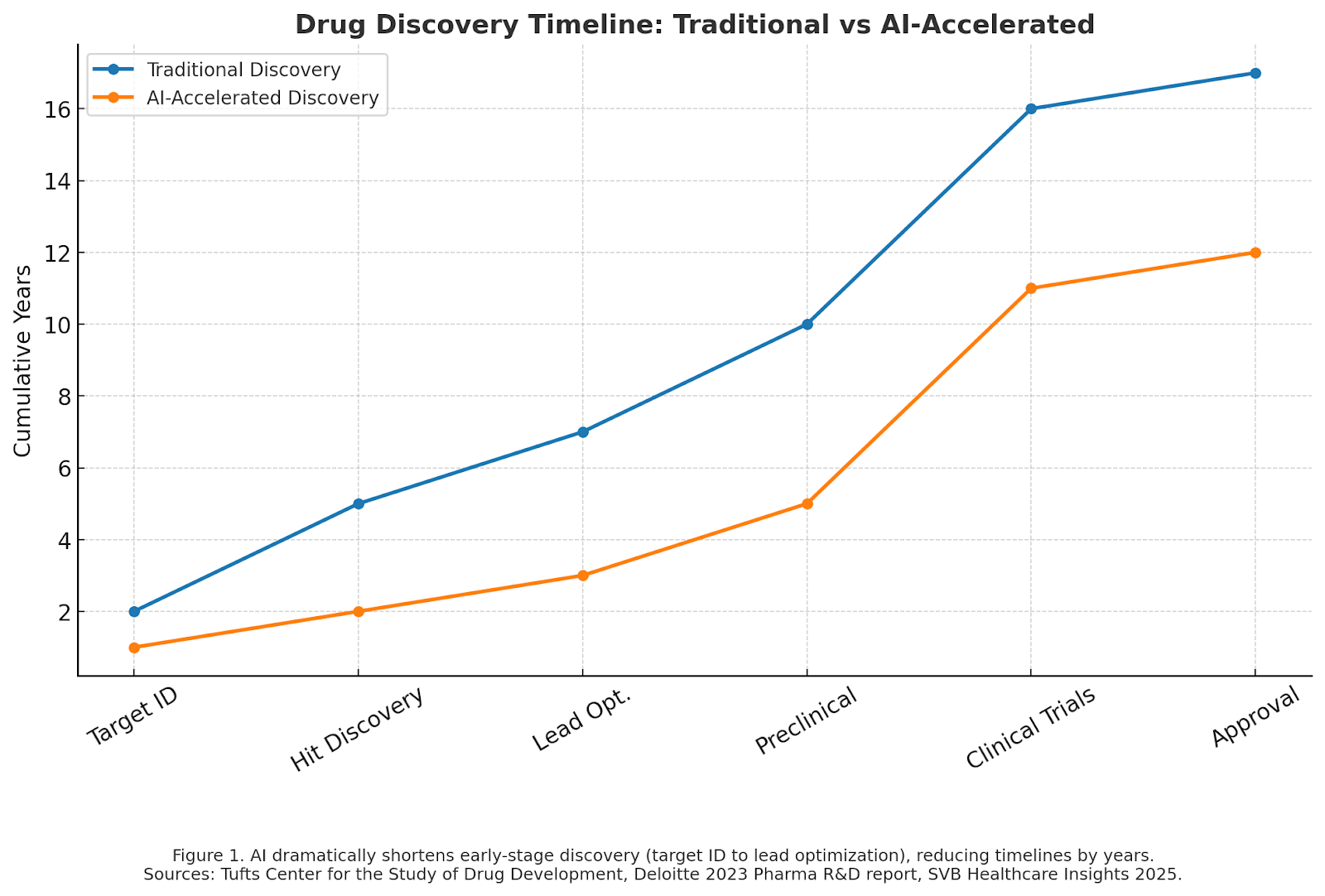
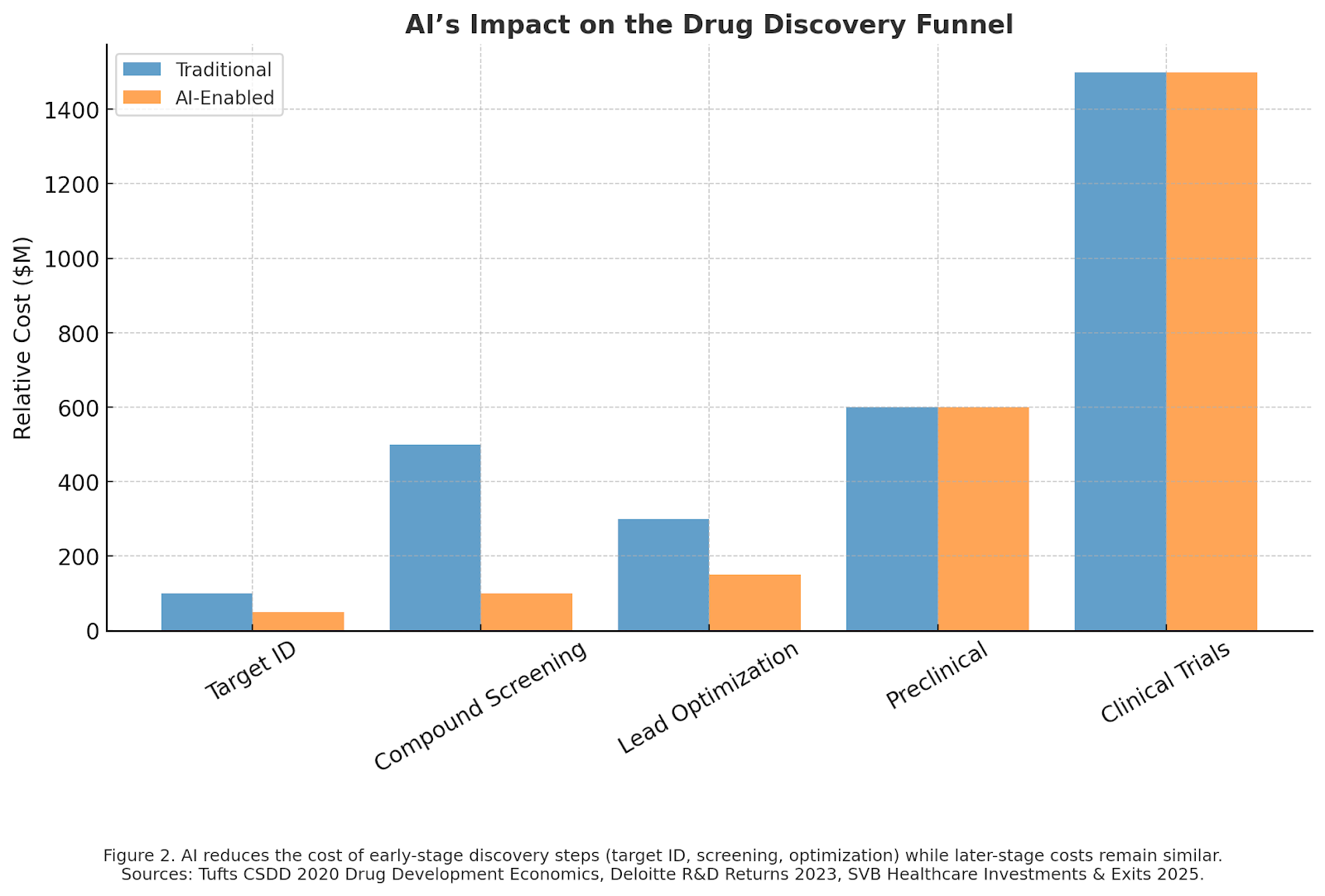
Traditional drug discovery is a marathon. On average, it takes 10–15 years and over $1 billion to bring a new therapy to market. Much of that time is consumed in iterative trial-and-error experiments. AI changes this equation by compressing discovery timelines.
- Virtual screening allows researchers to evaluate billions of molecular interactions in days, something impossible in wet labs.
- Predictive models reduce the number of compounds that must be synthesized and tested, speeding entry into preclinical and clinical phases.
- Automated lab robotics guided by AI can execute parallel experiments at speeds far beyond human capacity.
The net result is that discovery cycles that once spanned years can now unfold in months. For startups, this acceleration can mean hitting value-creating milestones before their capital runs dry—an existential difference in early-stage biotech.
2. A New Era of Precision and Personalization
One of AI’s superpowers is its ability to integrate vast, messy datasets—genomic sequences, proteomic maps, electronic health records, imaging scans—into coherent models. This enables levels of biological precision that were previously out of reach.
In oncology, for instance, AI models can predict how different tumor subtypes will respond to specific compounds, guiding personalized treatment strategies. In diagnostics, algorithms are being trained to detect subtle molecular signatures that signal disease years before symptoms appear.
This trend toward precision has several ripple effects:
- Smaller, more targeted clinical trials with higher success probabilities.
- Therapies designed for narrowly defined patient populations, supported by companion diagnostics.
- Reduced failure rates in late-stage trials, where the majority of drug development dollars are typically lost.
In other words, AI is pushing life sciences toward a world where biology is not only faster but smarter.
3. The Democratization of Discovery
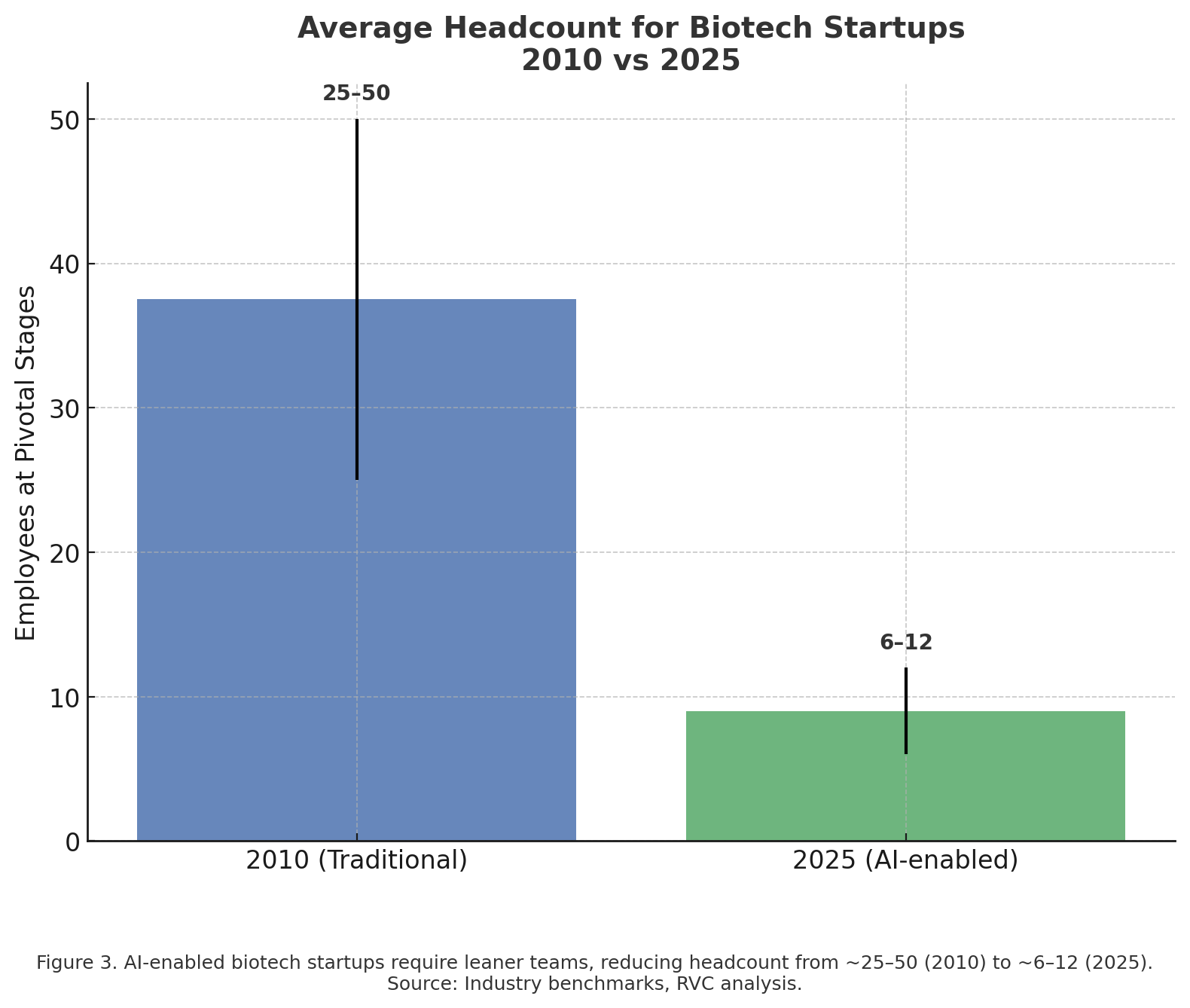
Historically, breakthrough science has required billion-dollar laboratories and armies of researchers. AI is lowering those barriers. Cloud-based platforms now allow small biotech startups—or even academic labs—to access AI-driven drug discovery tools on demand.
For example, companies like Atomwise and BenevolentAI provide AI-as-a-service platforms that can be rented rather than built in-house. This democratization means early-stage ventures can compete in areas once reserved for Big Pharma.
The implication: innovation is no longer gated by infrastructure, but by creativity and data access. This could spark a Cambrian explosion of small, nimble companies tackling niche diseases and rare conditions, fueled by AI rather than wet-lab armies.
Is AI Replacing the Lab?
It would be premature to declare the end of Petri dishes and test tubes. Biology is messy and often defies even the best computational models. Many AI predictions still require wet-lab validation, especially when moving into living systems. Moreover, regulators like the FDA require empirical evidence—not just in-silico simulations—for clinical approval.
That said, in areas such as protein folding, molecular docking, and toxicity prediction, AI has already surpassed traditional lab approaches in speed and sometimes in accuracy. Instead of replacing biology outright, AI is becoming the first lab—the place where ideas are tested, refined, and de-risked before ever touching a pipette. Wet labs are increasingly serving as the second lab, validating the AI’s insights and moving promising candidates into the clinic.
This shift is not replacement, but re-ordering. The sequence of discovery is being flipped, with computation leading and biology confirming.
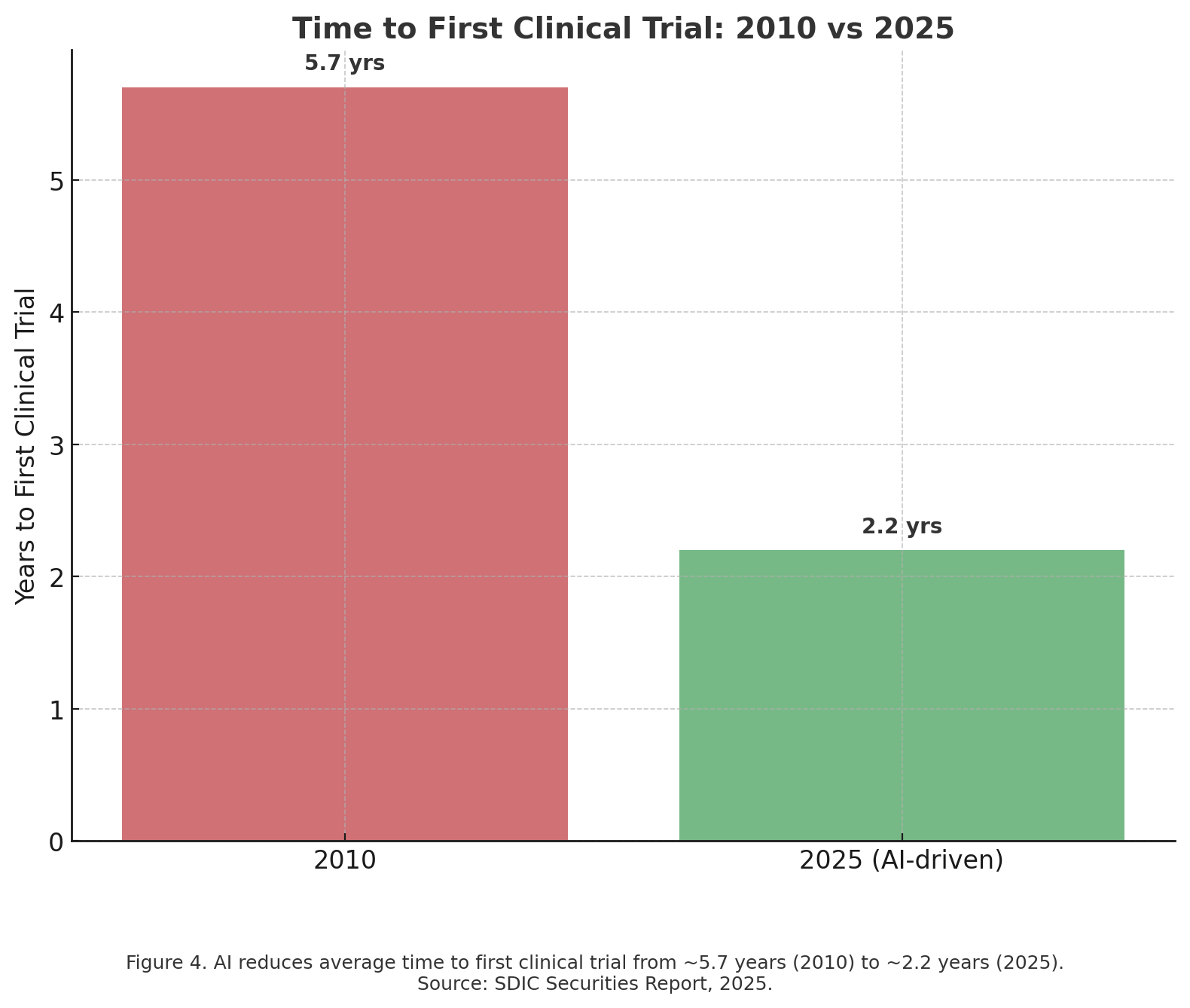
Here we see the current benefits of AI in early stage of research, but a smaller impact in clinical trials. In-silico models excel in prediction, but living organisms (mice, primates, humans) introduce complexity that AI can’t fully capture. For safety and regulatory reasons, animal studies and human trials are mandatory, regardless of how confident the computational models are. AI can help design better experiments (choose the right dose, stratify patients, predict toxicity risks), but it can’t replace the need to actually see how a compound behaves in a living body.
Regulatory Requirements Don’t Shrink
Agencies like the FDA, EMA, and PMDA require empirical evidence, not just simulations.
Preclinical toxicology, pharmacokinetics, and multi-species studies are still required for safety clearance.Clinical trials have minimum enrollment sizes, endpoints, and durations set by regulators. For example, even if AI suggests a drug will work in 3 months, a Phase III trial might still need to run 2–3 years to capture survival data.
Patient Recruitment and Trial Operations Are Human-Bound
The slowest bottlenecks in clinical trials are often logistical, not scientific:Recruiting the right patients,coordinating trial sites, fnsuring compliance and follow-up, gathering and cleaning real-world data.AI can improve recruitment (matching patients via EHRs), optimize site selection, and flag anomalies in trial data — but it can’t eliminate the fundamental human timeframes involved in trial conduct.
Endpoints Require Time to Emerge
Many therapeutic areas require long observation periods to measure outcomes (e.g., oncology survival, Alzheimer’s cognitive decline, cardiovascular events).No algorithm can “fast-forward” patient biology — regulators and physicians need to see how outcomes unfold over real time.
Implications for Early-Stage Life Science Investors
For venture capitalists and angel investors, the fusion of AI and lab science is reshaping the risk-reward equation in three ways:
- Shorter Timelines, Faster Milestones
AI-driven startups can reach proof-of-concept or preclinical candidates much faster than traditional biotechs. For investors, this means quicker value inflection points and potentially earlier liquidity events. However, it also means startups must scale quickly to stay ahead of competitors with similar tools. - Shifting Capital Needs
Whereas traditional biotechs required massive upfront infrastructure investments, AI-enabled companies can often operate with leaner budgets by renting compute resources and lab services. This changes capital allocation: more money goes toward talent and data access than bricks and mortar. Investors may see smaller seed rounds but larger follow-on raises once clinical validation begins. - Blurred Boundaries Between Tech and Bio
Investors must now evaluate companies that straddle two domains: computational AI and experimental biology. Due diligence requires understanding both algorithmic sophistication and biological plausibility. The winners will be those that integrate seamlessly—where the AI is not just a tool, but a co-scientist.
Conclusion: The Future of the Intelligent Lab
We are standing at the threshold of a new paradigm in science. AI is not a sidekick in the lab; it is fast becoming the laboratory itself. It accelerates discovery, enables precision, and democratizes innovation in ways unimaginable even a decade ago. While test tubes and Petri dishes will not disappear anytime soon, their primacy in discovery is already being challenged by algorithms capable of modeling biology at scale.
For early-stage life science investors, this convergence is both a promise and a challenge. The promise lies in startups that can deliver data-driven breakthroughs faster and cheaper than ever. The challenge is separating genuine integration of AI and biology from mere hype. As always in venture capital, timing and discernment are everything.
The lab of the future may be less glassware and more software. For those who place their bets wisely, the rewards could be transformative—not only for portfolios, but for patients whose lives depend on the therapies AI will help deliver.






.jpg)




